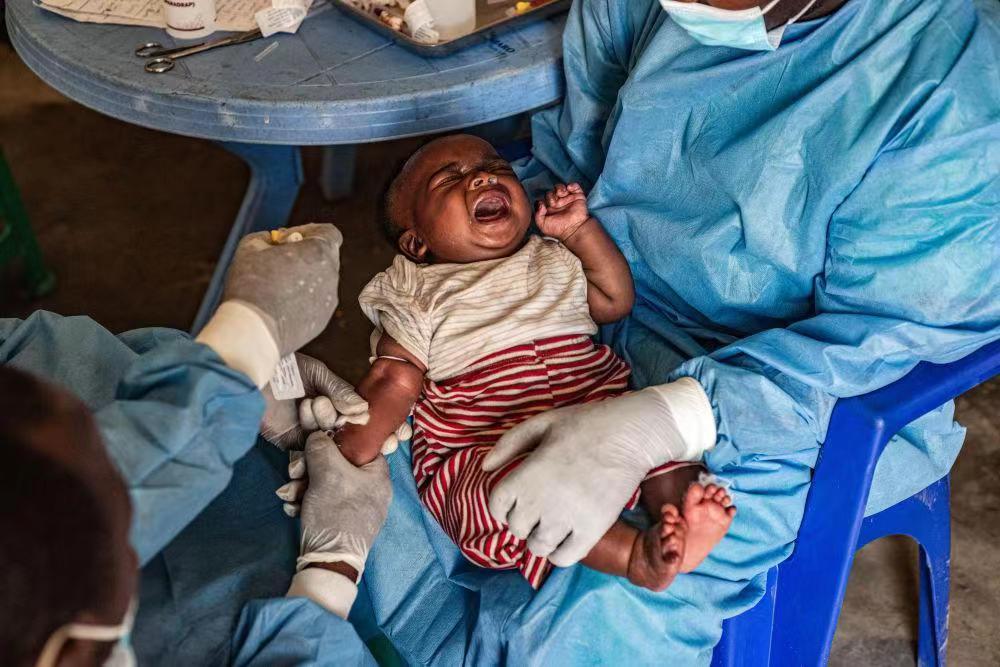
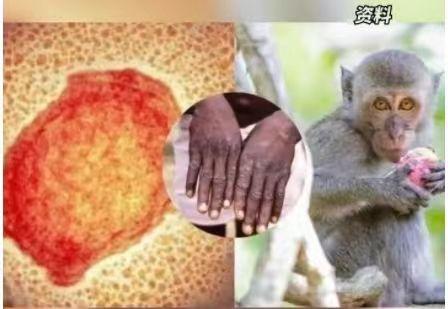
On August 18th, the National Medical Products Administration approved a monkeypox virus nucleic acid detection kit (QPCR)developed by a company in Guangzhou. This is the first monkeypox virus detection product approved for market in China. Monkeypox is a viral zoonotic disease caused by the monkeypox virus, characterized by fever, rash, and swollen lymph nodes. Historically, cases have primarily occurred in Central and West Africa, with a mortality rate ranging from 1% to 10%.
According to the World Health Organization (WHO), over 15,600 monkeypox cases have been reported this year, surpassing last year's total, of537 deaths. Recently, WHO Director-General Tedros Adhanom Ghebreyesusdeclared the monkeypox outbreak a "public health emergency of international concern (PHEIC)." This is the second highest-level alert issued by the WHO for the monkeypox outbreak since July 2022, drawing significant international attention.
In September of last year, monkeypox was classified as a Category B infectious diseasein China, with established procedures for identification, reporting, and isolation. Recently, the General Administration of Customsissued a notice to prevent the introduction of monkeypox into the country. The notice advises individuals arriving from countries or regions with monkeypox outbreaks to report to customs if they have had contact with monkeypox cases or experience symptoms such as fever, headache, back pain, or muscle aches.
It is noteworthy that many publicly listed companies in China are actively preparing for monkeypox. Several A-share listed companies have been involved in developing monkeypox detection. Notably, to meet the demand from international markets, products developed by companies such as Sansure Biotechand Da'an Genehave already received CE certification from the European Union and have been successfully exported to multiple countries.
This detection kit is also the first monkeypox virus detection product in China to be approved for market. It was developed by Da'an Gene, a high-tech company specializing in in-vitro diagnostics based in Guangzhou and listed on the Shenzhen Stock Exchange in 2004.
Source :Lingnan on the Cloud
国内首个猴痘病毒检测产品获批上市,由广东企业自主研发
8月18日,国家药品监督管理局批准了广州一家自主研发的“猴痘病毒核酸检测试剂盒(荧光PCR法)”,这是国内首个获批上市的猴痘病毒检测产品。猴痘是由猴痘病毒感染所致的一种病毒性人兽共患病,临床表现为发热、皮疹、淋巴结肿大。其既往病例主要发生在中非和西非,病死率为1%~10%。
世卫组织数据显示,今年以来报告猴痘病例数超过1.56万例,已超过去年病例总数,其中死亡病例达537例。近日,世界卫生组织总干事谭德塞宣布,猴痘疫情构成“国际关注的突发公共卫生事件”,这是世卫组织自2022年7月以来第二次就猴痘疫情发出最高级别警报,引起国际社会高度关注。
去年9月,猴痘被我国纳入乙类传染病管理,在识别、上报和隔离措施方面都已经比较完善。海关总署也于近日发布关于防止猴痘疫情传入我国的公告,提醒来自猴痘疫情发生国家(地区)的人员,如接触过猴痘病例或出现发热、头痛、背痛、肌痛等症状,入境时应主动向海关申报。
值得注意的是,当前,中国不少上市公司正在积极“备战”猴痘。多家A股上市公司均在猴痘检测方面有所布局,其中,为及时响应海外市场的需求,此前,圣湘生物、达安基因等研发的产品已经获得了欧盟的CE认证,顺利出口海外多个国家。
这款测试剂盒也是国内首个获批上市的猴痘病毒检测产品,该产品的研发企业为达安基因,是一家总部位于广州的体外诊断高新技术企业,于2004年在深交所上市。
文|记者 陈泽云
译|林佳岱
英文审校|王枥焓
-
Poster丨2024 Guangzhou's Top Ten "Lion Dance Youth Master" Crowned
2024-08-21 23:36:33 -
Qingyuan Lianshan: 'Three-land Revitalization'unlocks land potential, turning common mountain products into 'golden seeds' of prosperity
2024-08-22 00:06:55 -
ThePoint丨The thief crying 'stop thief' 贼喊捉贼
2024-08-22 00:06:55 -
'Black Myth: Wukong' propels Chinese culture onto the global stage
2024-08-22 00:06:55